Amid the Covid-19 pandemic, news of mRNA vaccines spread across the world. Put plainly, mRNA vaccines teach our bodies how to create important proteins, triggering immune responses that can shut down viruses. But this healthcare technology isn’t just for Covid-19.
Applications of mRNA medicines can include everything from the annual flu to insect-borne viruses to cancer and rare genetic disorders. Creating the blueprint is just one part of a multi-stage, complex journey to the patient.
Manufacturing and producing these novel therapeutics, such as the large-scale, multi-billion doses of the Covid-19 mRNA vaccine, requires cutting-edge technology, extensive research, and nearly flawless execution.
The complex manufacturing process for developing the Covid-19 vaccine was not an easy feat. In the past, the development and delivery of a drug or vaccine to the public would take at least a decade. We did it in less than a year, thanks to today’s incredible healthcare innovations. We see this new era of precision medicine as a core part of world infrastructure and support during the pandemic.
We now know what is possible in the future of healthcare technology, and scientists won’t look back. These complex and incredible applications point to the growth to come in the industry, and, as both patients and investors, we couldn’t be more optimistic.

Source: Lonza
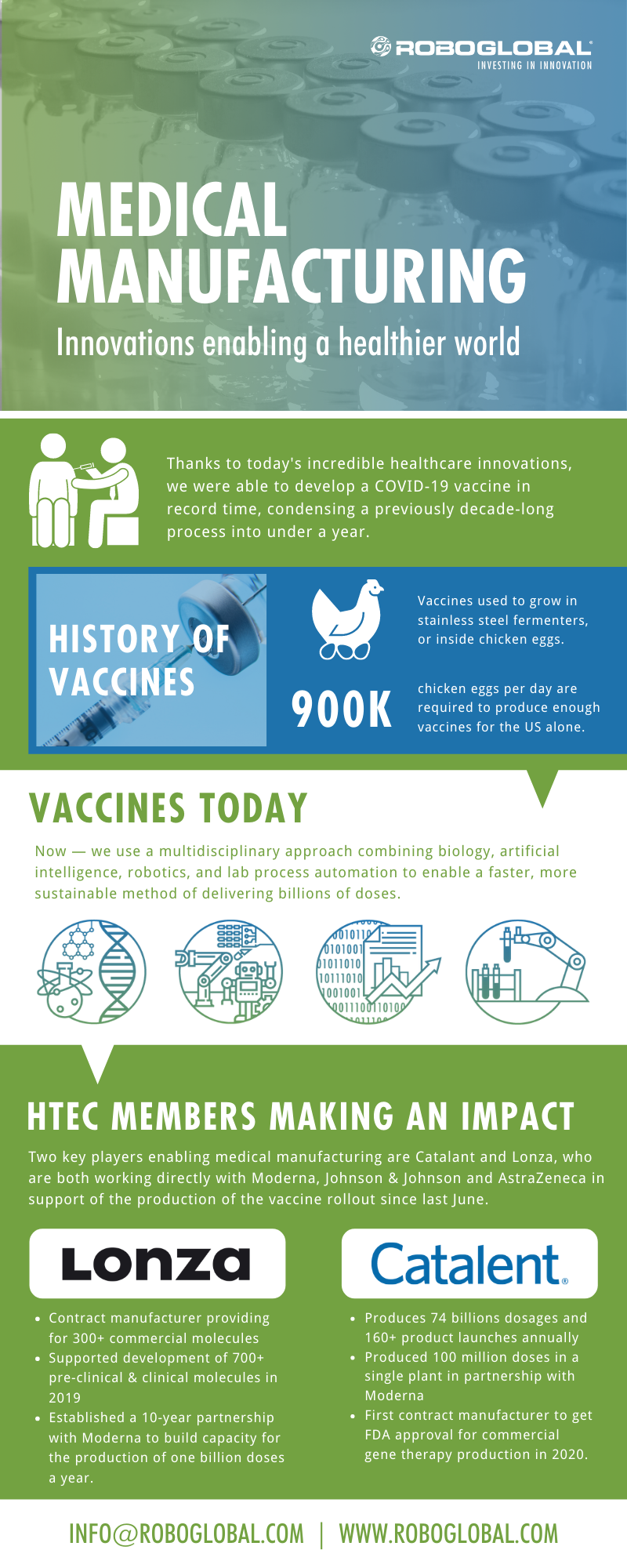
The Past & Future of Vaccine Manufacturing
Traditional vaccine manufacturing involved growing the vaccines directly within stainless steel fermenters, or inside chicken eggs. In fact, according to Rick Bright, former chief of the Biomedical Advanced Research and Development Authority, an astonishing 900,000 chickens eggs per day are required to produce enough vaccines for the US alone.
Now, we have a multidisciplinary approach that combines biology, artificial intelligence, robotics, and lab process automation. This is enabling a faster and more sustainable method of delivering billions of doses.
HTEC Index Members Making Enabling Medical Manufacturing
Catalant and Lonza are two HTEC index members that are working directly with Moderna, Johnson & Johnson, and AstraZeneca to support the production of the vaccine rollout since last June.
Swiss-based company Lonza is a contract manufacturer providing for more than 300 commercial small and large molecule drugs. They also supported the development of more than 700 pre-clinical and clinical molecules in 2019. Since its inception, thousands of compounds have been developed, thanks to its industry-leading expertise. Most recently, Lonza established a 10-year partnership with Moderna to build capacity for the production of one billion doses a year. Scaling these plants requires major investment, and Lonza recently announced intent to deploy an additional $935 million into two plants: one in their home base of Switzerland and another in New Hampshire. These extremely high-tech, automated labs will span large-scale manufacturing and smaller-scale bioreactors for future bio-manufacturing. Not only is Lonza seeing double-digit earnings growth, but also a solid net margin expansion from 13% in 2019 to its target of 17% this year.
Catalent is a global provider of delivery technologies and a leading drug manufacturer of biologics, gene therapies, and consumer health products. Catalent produces 74 billion dosages and 160+ product launches annually. Recently, Catalent announced an extension of their partnership with Moderna to rapidly fill sterilized syringes with the vaccine and have already reached the milestone target of producing 100 million doses in a single plant in Bloomington, Indiana. See the process in action here: Catalent Bloomington, IN - Syringe Filling Line).
An even bigger opportunity in the longer term for Catalent is the ramping up of their Cell & Gene Therapy suite. With the recent acquisition of Plasmid DNA, Catalent will be adding necessary cryogenic storage in the US and have new commercial-scale facilities on track to open next year. This will help to support novel gene therapies and precision medicines from the pre-clinical, trial stage to full global production. In fact, they were the first contract manufacturer to get FDA approval for commercial gene therapy production in 2020.

Source: Lonza
These innovative companies are transforming the way drugs are being manufactured and play a critical role in the development of groundbreaking therapeutics. Such incredible capabilities constitute just one of the many healthcare technologies that comprise our healthcare technology and innovation index. We anticipate that the development of mRNA vaccines and medicines will only continue to accelerate in the next decade, bringing us one step closer to personalized treatments and preventative care.